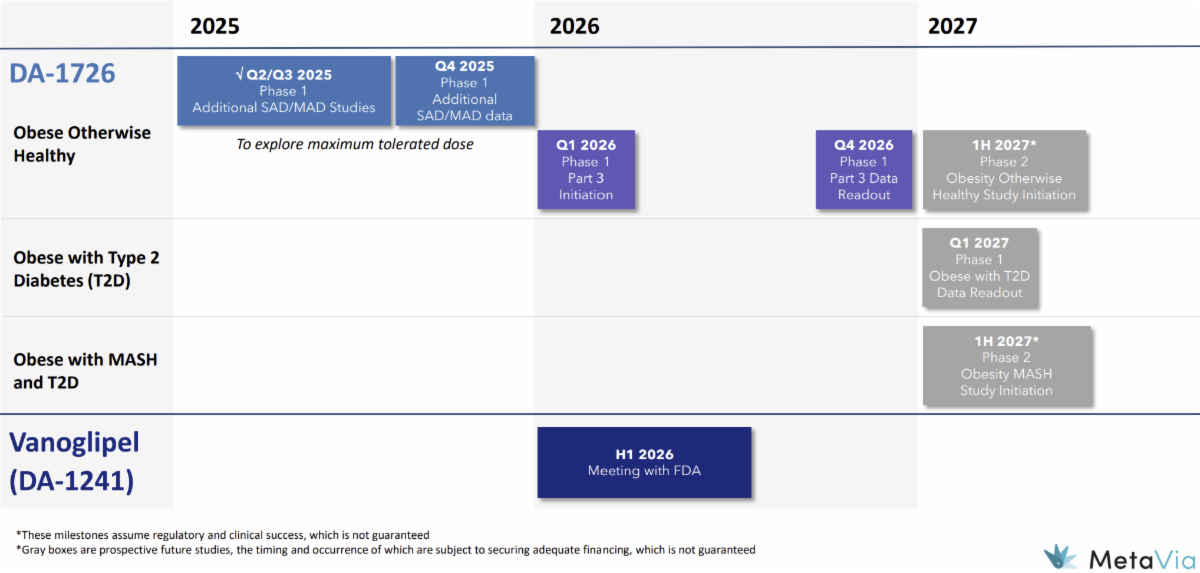
| DA-1726
Obesity is a complex disease in which excessive body fat increases due to nutrient imbalance. Obesity is a medical problem requiring interventional treatment to manage and minimize the risk of other heal-th problems, such as diabetes, high blood pressure, and heart disease, etc.
DA-1726 is a long acting oxyntomodulin peptide analog that can bind and activate both glucagon-like peptide-1 (G-L-P-1) and glucagon receptors. Activation of G-L-P-1 and glucagon receptors suppresses appetite and reduces nutrient intake.
In addition, activation of glucagon receptors in adipose tissue elicits fat burning. Thus, DA-1726, designed as a well-balanced dual agonist, induces wei-ght loss while effectively improving body composition.
Moreover, hypoglycemic effects induced by G-L-P-1 receptor activation were maintained. DA-1726 has a well understood mechanism of action, and, in pre-clinical mice models, resulted in improved wei-ght loss compared to semaglutide (Wegovy®) and cotadutide (another OXM analogue).
Additionally, in pre-clinical mouse models, DA-1726 elicited similar wei-ght reduction, while consuming more food, compared to tirzepatide (Zepbound®) and survodutide (a drug with the same MOA), while also preserving lean body mass and demonstrating improved lipid-lowering effects compared to survodutide.
In the Phase 1 multiple ascending dose (MAD) trial in obesity, the 32 mg dose of DA-1726 demonstrated best-in-class potential for wei-ght loss, glucose control, and waist circumference reduction.
Vanoglipel (DA-1241)
Vanoglipel (DA-1241) is a novel G protein-coupled receptor 119 (GPR119) mainly in the pancreas, intestine, and liver.
In non-clinical studies, GPR119 activation by vanoglipel in hepatocytes, macrophages, and hepatic stellate cells inhibits lipid accumulation, immune cell infiltration, and the production of collagen fibers in the liver, directly ameliorating MASH pathophysiology such as steatosis, inflammation, and fibrosis.
Moreover, GPR119 has a distinctive role in glucose and lipid metabolism via stimulating secretion of insulin and glucagon-like peptide-1 (G-L-P-1) in pancreatic beta cells and intestinal L-cells, respectively.
Therefore, vanoglipel can also provide additional metabolic benefits in MASH patients with common comorbid metabolic diseases such as type 2 diabetes and dyslipidemia.
In Phase 1b clinical trials, the safety and efficacy of DA-1241 monotherapy were successfully confirmed. Vanoglipel was well-tolerated in type 2 diabetic patients.
In a Phase 2a clinical study, vanoglipel demonstrated direct hepatic action in addition to its glucose lowering effects.
Uncover Sources And More Here: MTVA Website. MTVA Presentation. -----
5 Explosive Potential Catalysts Put (Nasdaq: MTVA) On Radar Watch
#1. MTVA Has A Really, Really Low Float (Volatility Potential Could Be Explosive).
With a float of roughly 981.54K shares, according to the Yahoo Finance website, volatility potential could pop up in a flash.
#2. A Potential Healthy Reversal Could Be Approaching For MTVA.
Technical analysis reveals MTVA has several oversold indicators, suggesting a possible reversal setup may be developing.
At close on Thursday, MTVA had a 9 and 14-Day Relative Strength Index both below 22% and a 9 and 14-Day Raw Stochastic below 4%.
These technical levels suggest a profile that may be currently undervalued.
#3. An Analyst Target From Zacks Small-Cap Research Suggests MTVA To Have Significant Upside Potential From Current Chart Levels.
Last month, Zacks Small-Cap Research analyst David Bautz provided MTVA with a massive target of $60.
From Thursday's close, that target represents a potential 3,500+% upside.
Here are some report highlights:
OUTLOOK
On January 5, 2026, MetaVia, Inc. (MTVA) announced positive and statistically significant results from the 8- week, non-titrated 48 mg cohort from the Phase 1b trial of DA-1726, the company’s dual oxyntomodulin analog that functions as a glucagon-like peptide-1 receptor (G-L-P1R) and glucagon receptor (GCGR) agonist. The results from that cohort showed a 9.1% (21.2 lbs) average wei-ght loss through Day 54 along with a statistically significant 9.8 cm reduction in waist circumference (P=0.022). The patients also demonstrated a 12.3 mg/dL improvement in fasting glucose from baseline along with a 23.7% reduction in liver stiffness. The company will next move on to a 16- week titration study, which we anticipate initiating in the first quarter of 2026 with topline results being available in the fourth quarter of 2026. -----
Furthermore, a Maxim Group analyst target of $55 is also massive.
From Thursday's close, that target represents a more than potential 3,200% upside.
#4. MetaVia Announces Positive AI-Modeling Results from its Ongoing Syntekabio Collaboration, Confirming Key Therapeutic Targets for Vanoglipel.
MetaVia reported promising results from its partnership with Syntekabio, where AI modeling using the DeepMatcher® platform validated key therapeutic targets for its GPR119 agonist, vanoglipel (DA‑1241).
The analysis confirmed strong engagement across inflammatory and cardiometabolic pathways, aligning with MetaVia’s focus on MASH and potential type 2 diabetes treatments.
These findings, alongside positive Phase 2a results showing improved liver and glucose function with good safety, strengthen MetaVia’s confidence in vanoglipel’s broader therapeutic potential and highlight the power of AI in its development strategy.
#5. MetaVia Reports Positive Statistically Significant Results from Its Phase 1b Clinical Trial of DA-1726 In Metabolic Disease - Demonstrating Strong Glycemic Response, Significant Direct Hepatic Effects, Robust Wei-ght Loss and Favorable Safety Profile.
MetaVia reported positive Phase 1b results for DA-1726, showing strong wei-ght loss, metabolic benefits, and liver improvements over eight weeks.
Patients on 48 mg achieved 9.1% (21.2 lbs) weight reduction, 9.8 cm waist loss, 12.3 mg/dL fasted glucose reduction, and 23.7% lower liver stiffness by Day 54, with no treatment-related discontinuations and only mild to moderate GI events.
The randomized, placebo-controlled design and planned 16-week titration studies support MetaVia’s confidence in DA-1726 as a differentiated, potentially best-in-class obesity and metabolic disease therapy. -----
Coverage is now officially underway on MetaVia Inc. (Nasdaq: MTVA).
Updates will be out soon. Keep your eyes peeled.
All the best, Dane James Editor Market Pulse Today
(Remember: St-ock Prices Could Be Significantly Lower Now From The Original Dates I Provided.)
*MarketPulseToday.com (“MarketPulseToday” or “MPT” ) is owned by Thousand Sun Media LLC, MPT is not responsible for its accuracy. Make sure to always do your own research and due diligence on any day and swing profile MPT brings to your attention. Any emojis used do not have a specific defined meaning, and may be used inconsistently. We do not provide personalized in-vest-ment advice, are not in-vest-ment advisors, and any profiles we mention are not suitable for all in-vest-ors.
Pursuant to an agreement between Thousand Sun Media LLC and TD Media LLC, Thousand Sun Media LLC has been hired for a period beginning on 02/13/2026 and ending on 02/13/2026 to publicly disseminate information about (MTVA:US) via digital communications. Under this agreement, TD Media LLC has paid Thousand Sun Media LLC seven thousand five hundred USD ("Funds"). These Funds were part of the thirty five thousand USD funds that TD Media LLC received from a third party named JRZ Capital LLC who did receive the Funds directly or indirectly from the Issuer and does not own st-ock in the Issuer but the reader should assume that the clients of the third party own shares in the Issuer, which they will liquidate at or near the time you receive this communication and has the potential to hurt share prices.
Neither Thousand Sun Media LLC, TD Media LLC and their member own shares of (MTVA:US).
Please see important disclosure information here: https://marketpulsetoday.com/disclosure/mtva-depio/#details | 


No comments:
Post a Comment